Washington: Food and Drug Administration on Friday paved the way for children aged 5 to 11 to get the Covid-19 Pfizer vaccine.
Dosage Size of FDA Children – Only one third of the amount given to adolescents and adults – for emergency use – for emergency use and up to 28 million more American children can qualify for vaccination as early as next week.
Another fixed regulatory obstacle: on Tuesday, advisory control centers and prevention of diseases will make more detailed recommendations where young people must be vaccinated, with the final decision by the agency director that is estimated shortly afterwards.
“With this vaccine the children can return to something better than being locked at home in a remote school, can’t see their friends,” Dr.
Kawsar Talaat from Johns Hopkins University.
“Vaccines will protect them and also protect our community.” Some countries have started using other Covid-19 vaccines in children under 12 years, including China, who have just started vaccinations for 3 year old children.
But many use vaccines made by Pfizer and the Bionech partners watch the decision A.S., and European regulators have just begun to consider the dose of the size of a subsidiary.
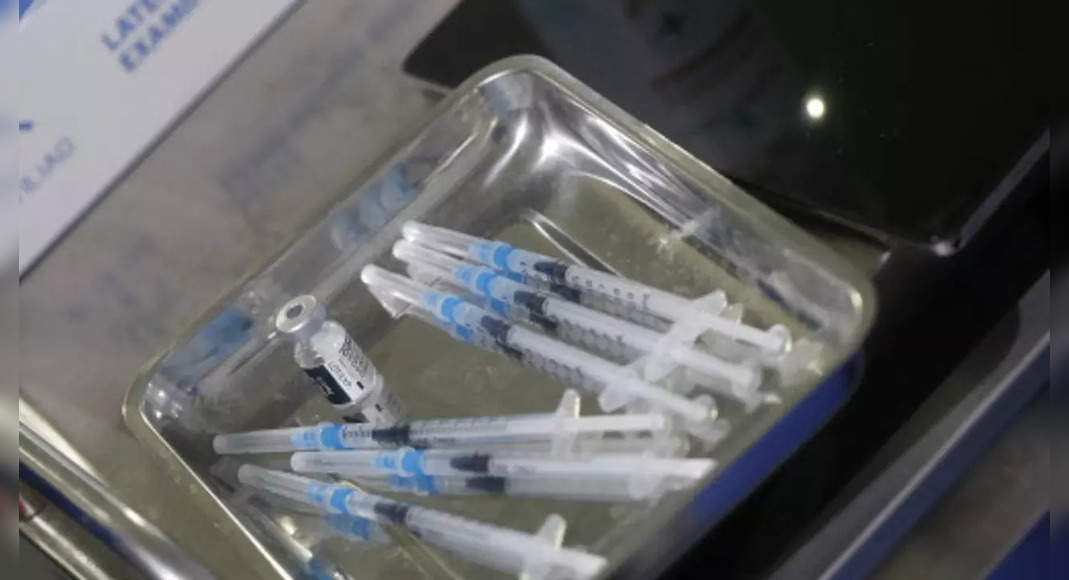
With the FDA action, Pfizer plans to start giving birth to millions of pediatric vaccine bottles – in orange close to avoid mixed with purple doses for everyone – to vaccination sites, other pharmacies.
Children will get two shots, three weeks apart.
While children at low risk severe diseases or deaths from Covid-19 rather than parents, aged 5 to 11 years are still affected seriously – including more than 8,300 inpatient care, about one third need intensive care, and almost 100 deaths since the commencement of the Coronavirus Pandemic , according to FDA.
And with an extra contagious Delta variant circulating, the government has calculated more than 2,000 closing schools related to Coronavirus since the beginning of the school year, affecting more than one million children.
Earlier this week, the FDA independent scientific adviser chose that the benefits of the pediatric vaccine promised to exceed any risks.
But some panelists say not all young people need to be vaccinated, and that they prefer shots targeted at those who are at higher risk of viruses.
Nearly 70% of 5 to 11 years hospitalized for Covid-19 in the US has other serious medical conditions, including asthma and obesity, according to federal tracking.
In addition, more than two-thirds of children who are hospitalized are black or Hispanic, reflecting the long gap in the impact of the disease.
The question of how the Pfizer vaccine is widely used will be the main consideration for the CDC and its advisors, which set formal recommendations for pediatricians and other medical professionals.
A Pfizer study of 2,268 school children found a vaccine nearly 91% effectively to prevent covid-19 symptomatic infection, based on 16 Covid-19 cases among children who were given a dummy shot compared to only three excavated.
The dose of the child also proved safe, with the same temporary or less reaction – like a sick arm, fever or pain – who experienced teenagers.
But this research is not large enough to detect very rare side effects, such as heart inflammation that sometimes occurs after the second full power dose, mostly in young men and adolescents.
It is not clear whether younger children get smaller doses will also face that rare risk.
Some parents are expected to vaccinate their children in front of the family vacation and winter winter meeting.
But the Kaiser Family Foundation survey recently showed that most parents were in no hurry to get a shot.
About 25% of parents surveyed earlier this month said they would make their children vaccinated “soon.” But most parents are roughly split between those who say they will wait to see how the vaccine performs and those who say they are “definitely” won’t have their children vaccinated.
Moderna vaccines made in the same way are also being studied in small children, and both Pfizer and Modera are also testing shots for infants and preschoolers.