Nagpur: The Department of Medical Public Medical College and Hospital (GMCH) will host Clinical Studies Phase III about the Corbevax e biological vaccine, the Covid vaccine developed by the original, especially in children in the 5-18 age group.
The trial will have 80 registration.
Children will be given two doses and booster doses after six months.
Registration is underway.
Nagpur is one of the 10 centers in this country for this trial.
Biological E is a company based in Hyderabad.
“Phase III clinical trials are underway in adults.
The results are very promising.
In GMCH, we are conducting clinical studies to find out how far he provides protection against Covid-19 disease in children,” said Dr.
Uday Narlawar, Hod from the Ministry of Medicine Community and major investigators for trials.
Registration for children will be carried out in a gradual approach to age with two subgroups.
Subgroup-1 will have teenagers from 12 to 18 years while subkroup-2 by having children from 5 to 12 years.
There will be two arms / treatment groups in this study which means half of the subject will be given a test vaccine while half a placebo will be given.
“Very challenging to get a subject for this research because of the strict exclusion criteria.
We appeal to parents to register them for this research,” said Dr.
Narwar.
Children who already have Covid-19 during the first or second wave and those who have lived in the same house as Covid-19 people will be excluded.
Children experience fever, history of blood disorders, asthma, chronic diseases will not be able to register.
Those who meet the requirements must provide written approval (by parents for minors).
They will be given a 0.5 mL vaccine dose through intramuscular injection.
Two doses will be given on days 0, day 28 and booster doses on day 208.
Sant Dnyaneshwwar Medical Education and Research Center, Pune, is the only other experimental site in Maharashtra.
Corbevax is a protein subunit vaccine.
The Ministry of Health and family welfare has been ordered in advance of 30 crore doses of Corbevax in June.
The source said that the vaccine will be launched for children voluntarily and will be available at a medical store with subsidized rates because the government bought it from the manufacturer.
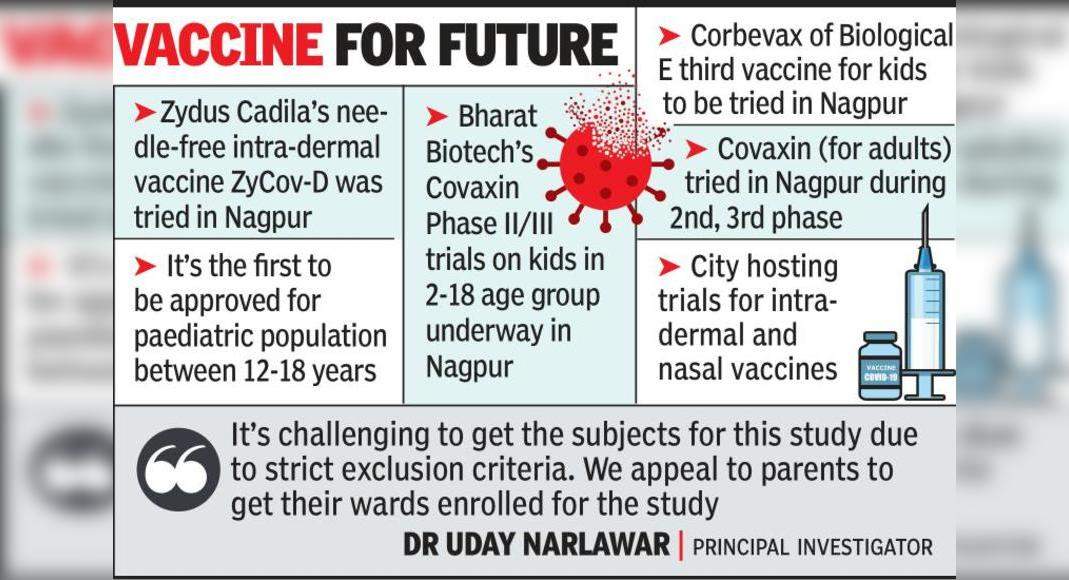
Vaccines for the Future – Intra-dermal Vaccine Zydus Cadila Zycov-D Needles Tried in Nagpur – This is the First Approved for Pediatric Population between 12-18 Years – Phase II / III Test of Bharat Biotech in Children at 2-18 The age of the bracket is also underway in Nagpur – Corbevax from the biological three vaccine for children to be tried in Nagpur – Covaxin (for adults) tried in Nagpur during the second and third phase – the city also conducts trials for intra-dermal and nasal vaccines on Covid-19