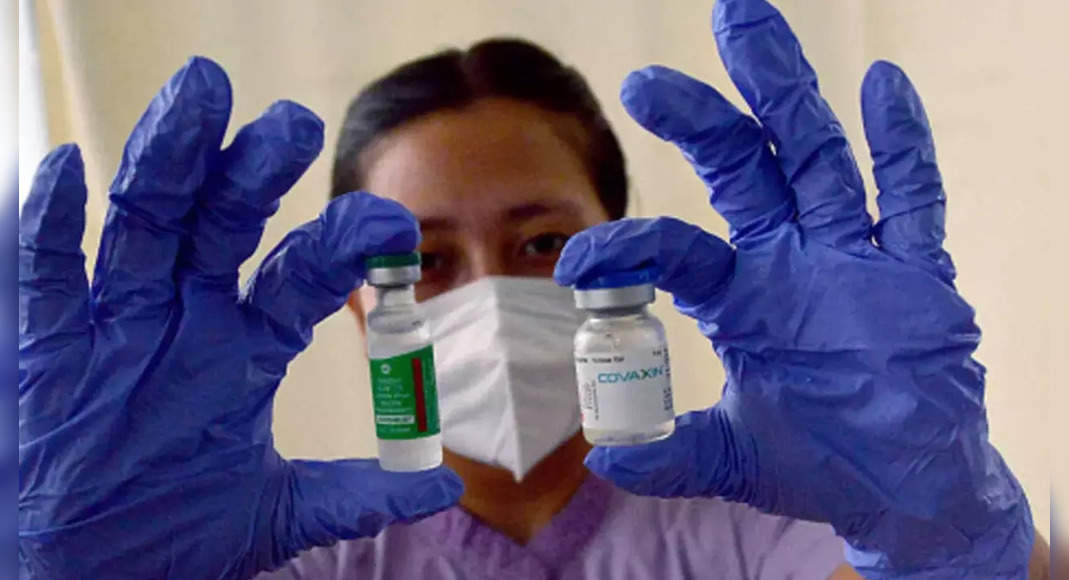
New Delhi: DCGI drug regulators on Thursday provide regular marketing authorization to two commonly used Covid vaccines – Covishield and Covaxin – to be used in certain conditions.
However, vaccines will only be available under “program settings” and all vaccinations must be recorded on Cowin.
This means even though vaccines will continue to be available in public and private centers registered under Cowin the Government, Jabs cannot be retailed at a pharmacy or chemist.
The two vaccines must submit data on the ongoing clinical trials from their formulations with maturity analysis every six months or earlier if available.
So far, both vaccines are under the authorization of emergency use.
While vaccines are given approval based on limited data from ongoing clinical trials, producers must submit safety data and side effects to regulators every 15 days.
With regular approval in place, manufacturing can now send the data once in six months.
While the government has not distributed details about the determination of the price of two vaccines, regular market authorization also allows the government to bring it below price regulations, said sources.
The Standard Control Center Control Committee of the Standard Organization has recommended the status of a vaccine from limited use in an emergency situation to provide new drug permits with conditions in the adult population on January 19, 2022.
“The latest approval provided by DCGI …
shows accuracy and accuracy The time with a public response strategy and state decision making equipment has responded to the needs that arise during a pandemic, “said the Ministry of Health in his statement.
“Conditional Market Authorization” is a new category of market authorization that has emerged during the current pandemic.
The approval path through this route is tracked quickly with certain conditions to increase access to certain pharmaceuticals to meet the needs of drugs or vaccines that appear.