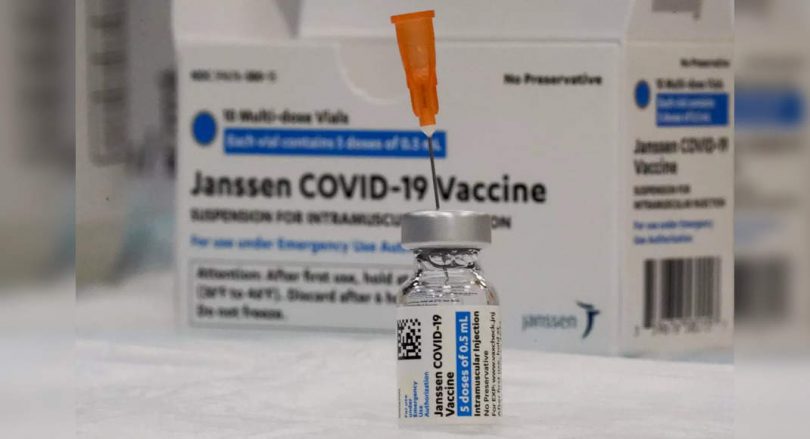
New Delhi: Johnson & Johnson has submitted an application to the Central Medicine Standard Control Organization (CDSCO) to conduct a study of the Covid-19 vaccine in India in adolescents aged 12-17 years.
Earlier last week, the Lovid-19 Dosage-19 vaccine Johnson & Johnson received an emergency approval of use in India, to become the fifth JAB against Coronavirus to get approval in this country.
However, there is an initial uncertainty of inventory as a problem around compensation for legal obligations if severe bad events have not been resolved.