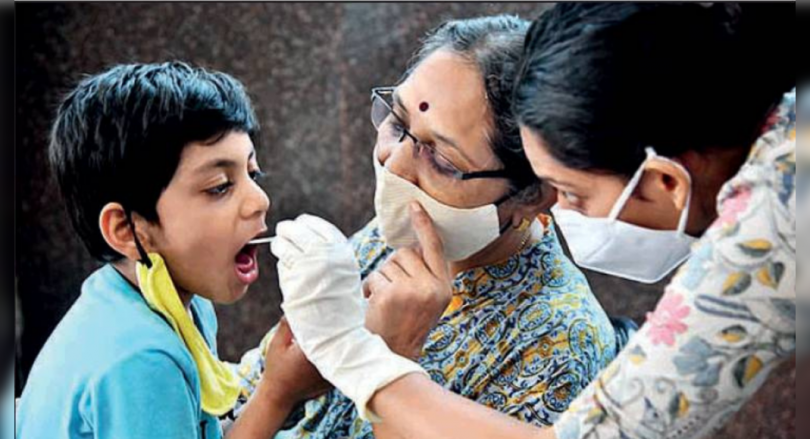
Mumbai: Phase II and III Phase Covaxin Test for current pediatric use and vaccines can be available for this age group in September or soon after, said Dr.
Priya Abraham, Director of the Institute of Virology (NIV) ICMR-National (NIV) Pune.
He said vaccination for children was widely awaited considering the fear that the third wave of potential could affect children in disproportionate.
In mixing vaccines and matching, he said studies in NIV did not find security issues and they would reveal more information.
The third wave can be avoided if people continue to cover and vaccinate as studies show that even with reduced efficacy of variants, vaccines work to prevent serious illness, Abraham said.
Q: How many covaxin trials in children advanced and when can vaccines be expected? Abraham: Phase II and III are active Covaxin trials for children between 2-18 years.
Hopefully, the results will be available soon.
They will be presented to the regulator.
So, in September or after that, we might have a Covid-19 vaccine for children.
This is separate, the Zydus Cadila vaccine trial also takes place for children.
Q: Apart from this, what other vaccines are in the pipe? A: The Zydus Cadila vaccine will be the first DNA vaccine available for use.
In addition, there is a M-RNA Gennova Biopharmaceuticals Ltd vaccine, biological vaccine, novovax institute serum and another interesting – intranasal vaccine by Bharat Biotech, which does not require Jab and can be sent through nostrils.
Q: Available vaccines that are effective on Delta-Plus variants? A: First, the Delta-Plus variant tends to spread than Delta, which is present in more than 130 countries.
In NIV, we have studied antibodies produced in people who were vaccinated and examined them against this variant.
The efficacy of antibodies to Delta reduces two three folds.
However, a protective vaccine against the variant.
They might show a little efficacy but it is important to prevent serious illness.
Q: Do we need a booster dose? Are there research conducted? A: Study has occurred abroad.
At least seven different vaccines have been tried for a booster dose.
Now, who stops it until more countries pursue vaccinations.
But, in the future, recommendations for booster will definitely come.
Q: What are the mixing and matching vaccines studied? A: There are situations where two different vaccines are given in two doses.
We have tested the sample in NIV and found that patients were safe.
There are no side effects recorded, and immunogenicity is a little better.
We are studying this phenomenon and will be able to provide further details within a few days.
Q: Mandiri testing kit has come to the market.
Will test testing? A: Mandiri testing kit is an antigen testing kit and then, the sensitivity is lower than the RT-PCR method.
Sensitivity is likely to be more in symptomatic patients.
Q: Can people infected with the bird flu virus or Zika become vulnerable to SARS-COV-2 infection? A: Bird Flu and Zika Virus are not related to Coronavirus.
But one similarity between Hini’s bird flu or swine flu virus and SARS-COV-2 is that their spread is prevented using masks, physical distance, hygiene and cough etiquette.
Zika spread through mosquito bites.
Q: Is it possible that there are no other waves to come? A: The new variant will continue to come.
We have two weapons cover and vaccination.
(Quotes from PIB interviews)