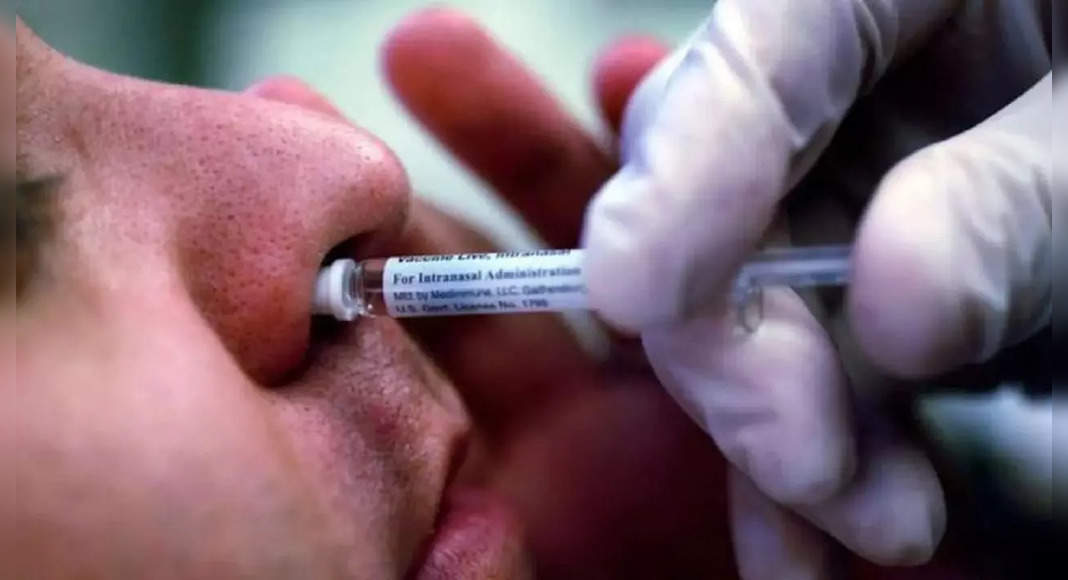
New Delhi: Medication Controller General India (DCGI) on Friday gave permission to Bharat Biotech to trial in an intranasal amplifier in India.
The trial will be carried out in nine different sites.
Hyderabad-based manufacturers have proposed a booster dose for those who have not been inoculated with covishield and covaxin vaccines.
According to a source, Bharat Biotech aims to conduct clinical trials at 5,000 healthy subjects: half or 2,500 individuals who have received the covishield and 2,500 others who have given Covaxin.
There will be a gap about six months between the second and intranasal booster dose.
The source said that the Nasal Booster vaccine was likely to be launched in India in March after the clinical trial.
In his speech to the country on December 25, Prime Minister Narendra Modi has assured that the state will soon develop a nose vaccine.
(With input from the agency)