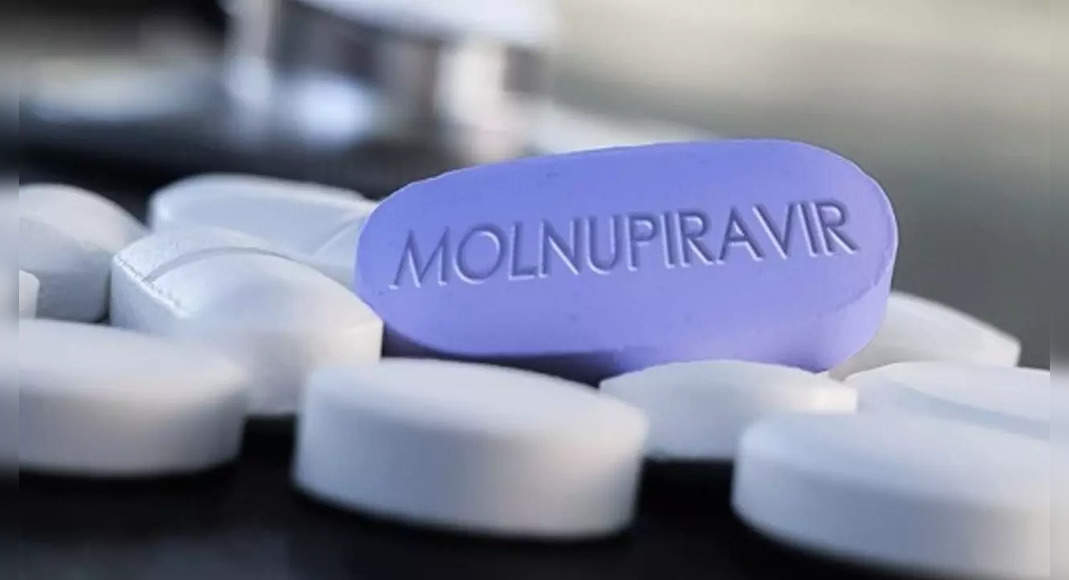
Pune: The State Health Department has decided to issue a series of new norms after drawing flares to enter new oral anti-virus pills, Molnupiravir, for more infected “non-comorbid” covid patients in the new revised guidelines revised on Thursday.
.
Pradip Kick, State Supervision Officer, Maharashtra Health Services, said, “The inclusion of Molnupiravir for minor non-commorbid patients in the new revised treatment guidelines is based on the initial pieces of the drug trial.
We will remove the drug for non-comorbidi in revisions Next immediately.
We will also disgrace doctors through our workshops so that the drug is not used irrational.
“Dr.
Archana Patil, Director, Health Services, cannot be contacted for his comments, even though it repeatedly.
Globally, including India, Molnupiravir is only approved for covid-19 patients who are light with the risk of high progress on severe diseases, especially those who have existing conditions (comorbid).
The Indian Medical Research Council (ICMR) has excluded it from Covid treatment which quotes serious safety problems / side effects and for possible drugs that are not irrational and change like steroids.
Critical Care Expert Dr.
Kapil Zirpe, a member of the Covid Task Force in Pune, “Nothing in this world is a new and cheap drug (Molnupiravir) which is permitted to be used for patients without comorbidity.
The inclusion of drugs in the revised treatment guidelines is not scientific and shows The lack of views ahead.
Inclusion for minor-infected patients without underlying medical conditions can cause misuse because many general practitioners and doctors take part in this guideline, and cases of covid light are increasing.
“Use of molnupiravir nuances.
“Until not, there is no evidence of use among mild diseases without comorbidity.
Even among those who are not vaccinated with a higher risk of development, the benefits are only simple – 30% reduction in hospitalization,” said a member of Sanjay Pujari, a member of the task force National covid.
In addition, Molnupiravir cannot be used in pregnant women and children.
State Care Guidelines (released on January 6, 2022) also stated that it should be avoided in pregnancy.
Pregnancy must be ruled out before advising drugs and women must practice contraception up to four days after completing the dose.
For men who accept Molnupiravir, contraception is recommended for three months due to mutagenicity concerns (capacity to induce mutations) and genotoxicity (causing damage to genetic material in cells).
“Other concerns include unclear efficacy of the Delta and Omicron variants and the potential to encourage mutations in the virus,” Pujari said, member of the task force.
Experts say the use of molnupiravir must be decided carefully after weighing the risk-benefit ratio.
Even in India, approval is only for mild diseases with developmental risk factors.
They have also advised the government to regulate the use of excessive drugs.
Medication, developed by biototapeutika biotecheback Biotechnology based in the US in collaboration with the Pharma As Merck giant, is being carried out by 13 Indian drug manufacturers.
It is commercially available and can be searched by a doctor’s prescription.
“Because the covid light is increasing, independent treatment with Molnupiravir is a big problem.
People often access drugs are included in their own care guidelines and treat themselves,” said Dr.
Sanjay Patil, Chair of the Indian Medical Association Hospital from India.