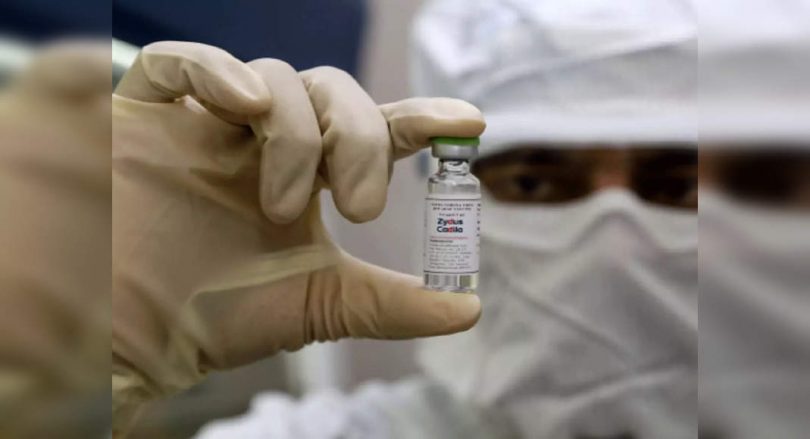
New Delhi: India is ready to launch the world’s first DNA-based covid vaccine that will be given to children over 12 years and also adults.
Zydus Cadila was developed by Jab Zycov-D which was developed in India on Friday for emergency use and the company planned the commercial launch around mid-September.
It will effectively become the fourth Covid vaccine available for mass use in India after covishield, Covaxin and Sputnik V.
Overall, six vaccines have been approved for use in India.
Here are the main things that need to be known about new Indian anti-covid shots …
* Unlike every other vaccine used, Zycov-D will have a 3-dose regimen with a 28-day interval between each shot.
All other anti-covid shots in India are two dose vaccines.
* Zydus shots will be given using a needle-free applicator that contradicts syringes.
It is being done to help reduce side effects.
* The temporary results of phase 3 indicate that Zycov-D has a primary efficacy of 66.6%.
The company separately said that this vaccine was 66% effective against the Delta variant, with a higher level of antibodies in the age group of 12 years and 18 years.
* Cadila is expected to supply around 5 doses of crore in December this year.
The company said she was planning to increase vaccine production to 1 dose of crore per month from October this year with the help of a new production plant.
* Vaccines can be stored at 25 degrees Celsius for 3 months.
* Vaccine prices are not yet known and are expected to be completed next week.
* DNA & RNA-based vaccines.
Wre they? Vaccines use genetic material parts of the virus that provide instructions as DNA or RNA to make specific proteins that the immune system recognizes and responds.
The technology of “plug-and-play” where plasmic DNA platforms are based can be easily adjusted to handle mutations.
Both the US-based moderna and pfizer are MRNA vaccines.
Covishield is a virus vector shoot that uses a modified and harmless virus to give instructions to the immune system while Covaxin uses an active actual virus strain.
(With input from agency)