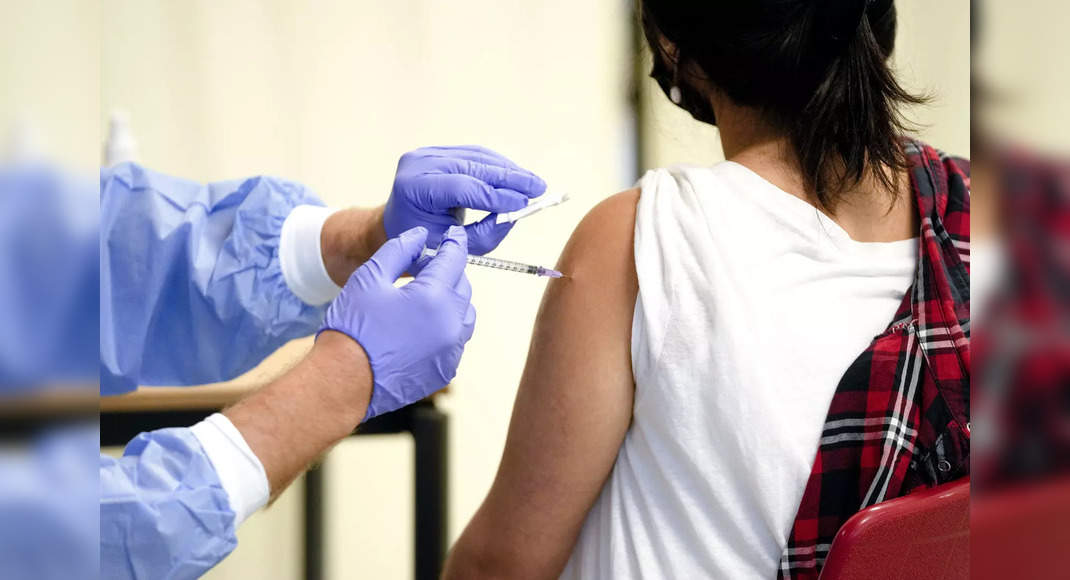
New Delhi: The government plans to start the covid vaccination of children in the age group 12-17 which have co-morbidity such as obesity, heart disorder, or immune efficiency in October-November, official source said.
The plan is to keep in mind that the supply of Zydus Cadila Zycov-D-DNA vaccine – the only one who has received emergency authorization for use in children in this country so far – is likely to begin in October.
“We are waiting for Zydus to start supplies.
After that it starts, we will start vaccinations among children this year, only those who have co-morbidity will receive a vaccine dose, the break will begin to get it from the first quarter of next year,” said A senior official told Toi.
Read the scientific body Alsono suggested covid vaccination children must be conditions to reopen the school: The government government said that there was no scientific body that showed that the vaccination of children against Covid-19 must be a condition to reopen the school, but teacher inoculation, school employees , and the parents want.
There are 20-30 children lakh with co-morbidities will be eligible for the first round of vaccination, according to government estimates.
Zydus is expected to supply around 40 lakh doses in the first lot, and scale up to one crore every month ahead.
The government expects that the company will be able to supply around 4-5 doses of crore in December.
Zycov-D is a three-dose vaccine.
The government hopes to expand further reach among children in March next year, when more vaccines are available for them.
Also read who prepared to activate the final evaluation vaccination of the country’s Childrenthe Pediatricians will review international data in children who are vaccinated against Covid-19 and present them to the National Technical Advisory Group on Immunization (Ntagi) early next week, reported economic time.
“The pediatricians will also be tested for children and clinical trials and clinical trials are also completed, according to regulatory officials.
After Covaxin gets an emergency authorization for use in children, it can be given to all those above two years.
Also another vaccine candidate from biological E and Indian Serum Institute which has recently received approval for clinical trials in children.
Overall, there is an estimated 44 crore children under 18 years old.
From this, around 12 crores are on The 12-17 year age group, which tends to be the first to receive a vaccine.
However, given the limited inventory, the government has decided to prioritize them with co-morbidity in this age group because they are vulnerable to infection.
The government also believes that the majority The adult population gets at least the first dose of Jab Anti-Covid, they can start with children-a Son – who is now more focused on the reopening of schools and other related activities is under locking since March 2020 because of the Covid-19 outbreak in this country.