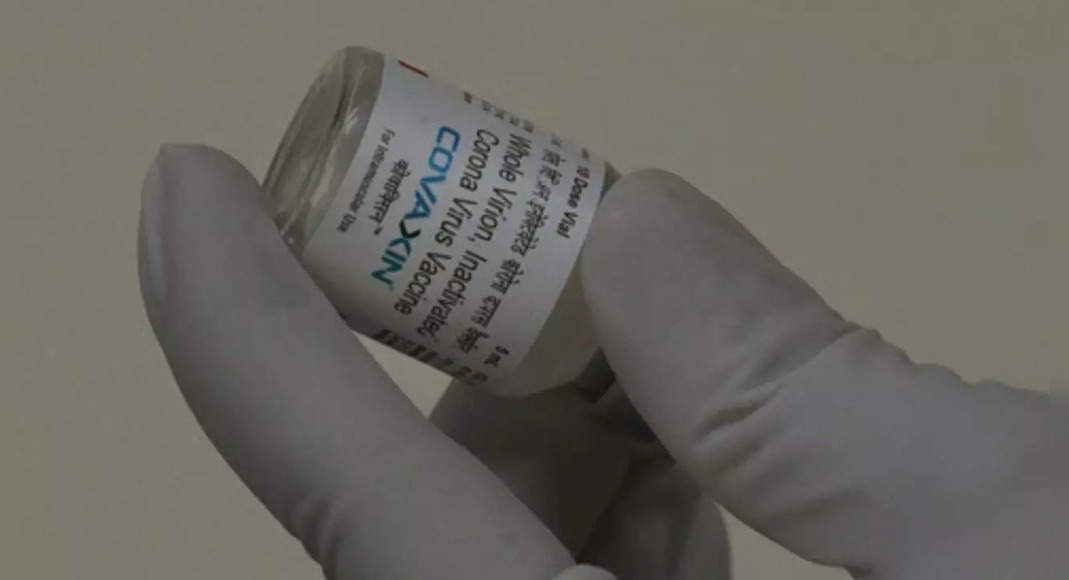
New Delhi: Technical Advisory Group (Tag), Independent Advisory Panel The World Health Organization (WHO), has been looking for additional clarification from Bharat Biotech, Covaxin manufacturer, to make a risk of listing end-emergency use (EUL) -Benefit assessment for the use of global vaccines.
The World Health Organization will meet on Wednesday for the final risk assessment for the list of emergency use.
“Tags meet on October 26, 2021, and decided to look for additional clarification from the manufacturer of Covaxin needed to assess the benefits of the final EUL risk for the use of global vaccines.” said an official.
The technical advisory group has met October 26 and has been looking for additional details from Bharat Biotech.
Covaxin has shown 77.8 percent effectiveness of Covid-19 and 65.2 percent simtomatic protection against new Delta variants.
In June the company said it concluded the final analysis of the efficacy of the Covaxin of the phase test 3.
Bharat Biotech’s Covaxin and AstraZeneca and the Covishield Serum Institute were two vaccines that were widely used in India.
Last week, WHO said that the period of time for the procedure for recording emergency use depends on how fast the manufacturing company that produces the desired vaccine is able to provide the data needed for whom will evaluate the efficacy, security, quality, and that is suitable for vaccines.
for low and medium-income countries.
Meanwhile, Indian cumulative vaccination coverage crosses 107 crores.
The WHO has a Covid-19 vaccine that is approved so far from Pfizer-Bontech, Astrazeneca-SK Bio / Serum Institute of India, Johnson 7 Johnson – Janssen, Moderna, and Sinopharm for emergency use.
(With input from the agency)