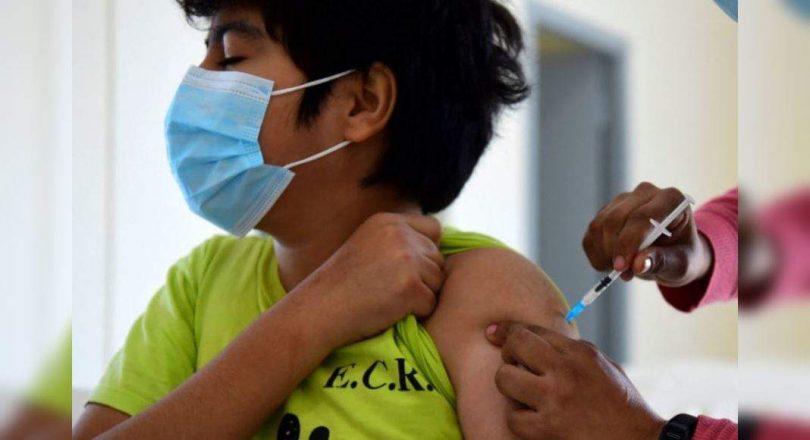
New Delhi: Trial for Vaccine Bharat Biotech Covid-19 Covaxin in children is underway and the results are expected to be released in September, Director of the All India Institute of Medical Sciences (AIIMS) Randeep Guleria.
“Vaccines for children must come out now because trials in India have been going on for vaccines available in India.
Bharat Biotech trials are in the final phase and in September we will have data,” Guleria Ani said.
He added, “In the coming weeks or in September, vaccines must be available for children.
We must start school in a multilevel manner as we have done for 18-45 years and it will also provide more protection to children And more trust to the public that children are safe.
“The trial is being carried out in three phases by separating children into the category according to their age.
The first trial began in the age group 12 to 18 years followed by the age group 6 to 12 years.
The trial for children between ages and 2-6 years is currently undergoing a trial.
He further said that the Zydus Cadila vaccine producer, who recently asked for an emergency use authorization for the Covid-19 Zycov-D vaccine, also entered data for children for their vaccines.
“The Zydus Cadila vaccine also includes children and the data already there.
They have applied for emergency use permits,” he said.
Zydus Cadila has concluded his trial for children between the age group 12 to 18 years.
The Ahmedabad-based pharmaceutical firm on July 1, requested an approval of emergency use for the Covid shot three doses – the first plasmid DNA vaccine in the world.
However, it will be a few more days before the drug control drug controller from India (DCGI) provides authorization of emergency use, according to the source.
The Indian government has held a discussion with the manufacturer of Covid-19 Moderna vaccine and Pfizer, to get their vaccines.
However, there is the same delay.
“There may be many reasons for delays.
I think two or three things to remember – someone is collaboration and understanding with the government at the dose.
They must have enough doses to be given because we must understand that these companies must understand the pre-order orders from many country, “he said.
They cannot let go of the dose for other countries unless they fulfill the obligations to countries that have been booking, Director AIIMS added.
India has discussed with two companies on several problems including the neglect of compensation.
Previously, Dr.
V K Paul, Member (Health), Niti Aayog said, “We held a discussion.
This is the process of negotiation and dialogue.
We try to get a solution to contract problems and commitment.
This process is underway.” Watch ‘Covaxin trial results in children who are likely in September’