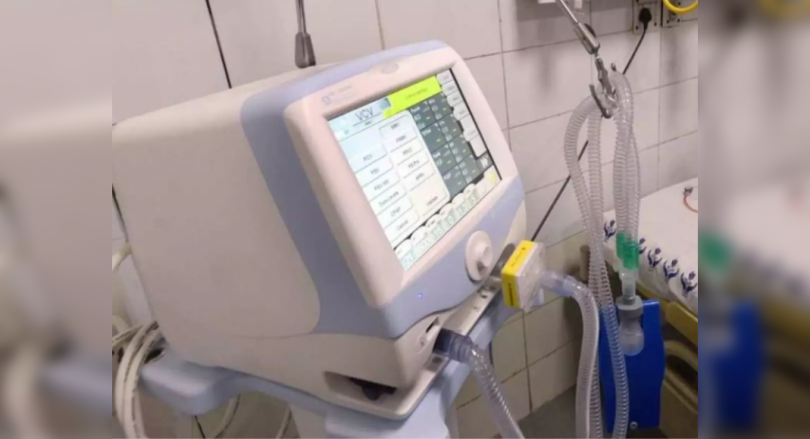
Chennai: Two months after Philips Medical Device maker announced the withdrawal of the Pressure of the Philips BI-Level (BI-Level Pap), continuous (CPAP), and a mechanical ventilator, the state government has asked the company to replace fix it.
Defects in at least 600 invasive and non-invasive ventilators used in government hospitals.
The company has issued safety warnings to overcome potential health risks including toxic and carcinogenic effects associated with the components used in this device.
On June 14, this initiated a remember notification.
This device pushes air to the lungs of people with problems such as chronic obstructive pulmonary disease or sleep disorders, where breathing stop intermittently.
Notifications say foam is used in this device to reduce sound and vibration damaged.
It can break down small particles and if inhaled, these particles can cause short and long-term health problems.
“The risk of potential exposure to gas-gases includes headaches, irritation, hypersensitivity, nausea / vomiting, and possible toxic and carcinogenic effects.
Philips does not receive reports on the impact of patients associated with chemical emissions,” he said.
However, fine print reads that remember notifications only for the US.
For the whole world it is only “notification of field security.” On August 9, the standard drug control organization issued a warning of medical devices stating that Philips India Limited “voluntarily issued field safety notifications” for the necessary improvements and the use of sustainable devices.
While warning foam can enter the airway device, he also warns that “high heat and a high humidity environment can contribute to foam degradation in certain areas”.
The foam can emit chemicals and gas during the initial operation and can continue throughout the useful life of the device, he said.
It suggests patients using the BI-Level Pap and CPAP devices to stop using this device and consult with their doctor.
Those who use mechanical ventilators have been asked not to stop using without consulting their doctor.
During the week, Tamil Nadu surveyed a device in all government hospitals.
“We have bought four of almost a dozen the wrong models during the period.
Our biomedical engineers are now reviewing them and also gather information about machines present in government hospitals throughout the country received as donations.
We ask the manufacturer for a replacement or take steps in need of fixing the problem “said Tamil Nadu Medical Services Managing Director Deepak Jacob.
This device is widely used during Covid-19 in patients with medium and severe illness.
“We have adequate breathing devices such as various high-flow ventilators and nose channels that can be used as an alternative to the wrong device.
Our engineers will ensure that there is no shortage of this equipment anywhere,” he added.
However, the state or company does not have a count on the number of people who use this device in private hospitals or at home people in Tamil Nadu.
One user, Padma Reddy 91 years old, a vepery resident, who has used a non-invasive ventilator at night at his home since 2018 for his COPD, complained of nausea last week.
After running a few tests, the doctor asked his family to serve the ventilator.
“That’s when a bio-medical engineer told us that the machine was wrong.
We put the tube on a white cloth and saw some small black particles,” said his daughter Vidya R, who is now waiting for Philips to take corrective steps.
While Padma was lucky to find a defect and find a replacement of the device, pulmonologist expert said many patients who used this device at home may still not be conscious.
“We signed up with the company as soon as they released a warning.
They have served 10 oxygen concentrators and three non-invasive ventilators.
Our machines are fine because they are well maintained,” said Dr Avinash Rajkumar from Nupre Out reaching medical services, which offers Home care services.
“But people who buy it for personal use may not be aware of Alter’s maintenance or safety,” he said.
A senior official from Philips told Ti that the company would replace foam with new materials on all the wrong devices.
“We have asked users to register devices on our website or contact us at 000800-852 1411 to register their device,” he said.