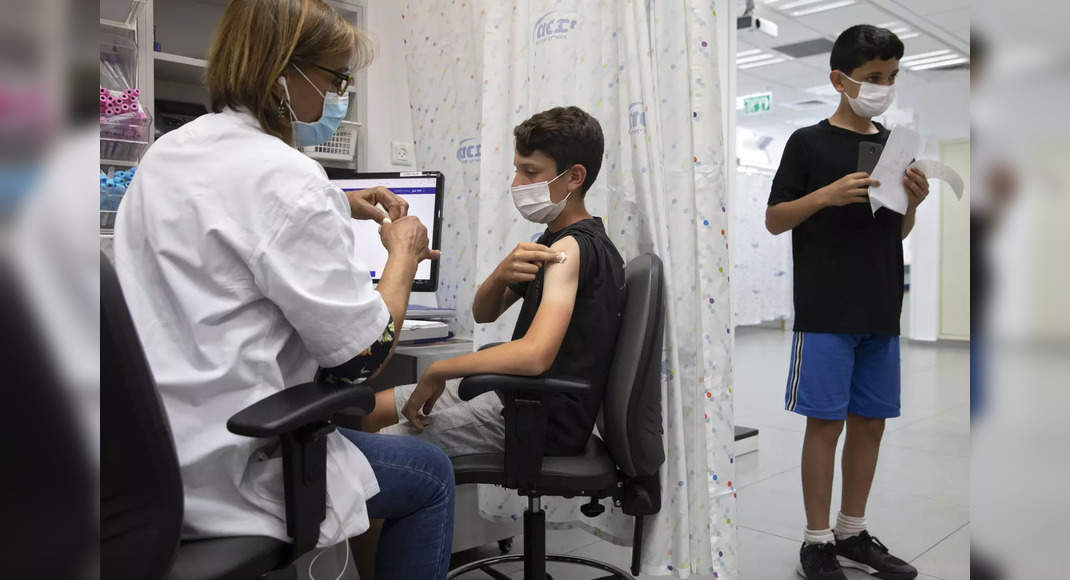
Advisors to the US Center for Disease Control and Prevention (CDC) on Tuesday unanimously support the use of Pfizer and Covid-19 bionech vaccines in children aged 5 to 11, with shots potentially on young arms as soon as Wednesday.
They say vaccination benefits are greater than the risk of vaccines.
Most of their discussions come from rare cases of heart inflammation that have been associated with vaccines, especially young men.
CDC Director Rochelle Walensky must sign a recommendation before the United States can begin managing vaccines to children in the age group.
The US Food and Narcotics Administration provides authorization of emergency use from vaccines at the age of 5-11 years on Friday.
The FDA ratifies a dose of 10 micrograms of Pfizer vaccines in small children.
The original shot given at the age of 12 and older is 30 micrograms.
At the beginning of Walensky’s meeting said that the pediatric hospitalization had surged during the recent wave driven by the Delta variant of Coronavirus.
The risk of Covid-19 “is too high and too awesome to our children and is much higher than many other diseases that we vaccinate children,” he said.
Walensky said the closure of the school has harmed the social impact and mental health in children.
“Pediatric vaccination has the power to help us change all that,” he said.
The US and Pfizer governments have begun distributing vaccines in preparation for expanding launches for children, many of them return to schools for direct learning.
Earlier this week, the White House said the United States had enough supply of PFIZER / BONALECH vaccines for all 28 million children aged 5 to 11 years.
While some children might get their first shot immediately after Wednesday, the plan is to plan the US pediatric vaccine program will run with full force next week, a Biden Administrative Officer said.
Only a few other countries, including China, Cuba and the United Arab Emirates, have so far have a covid-19 vaccine which is far away for children in this age group and younger.