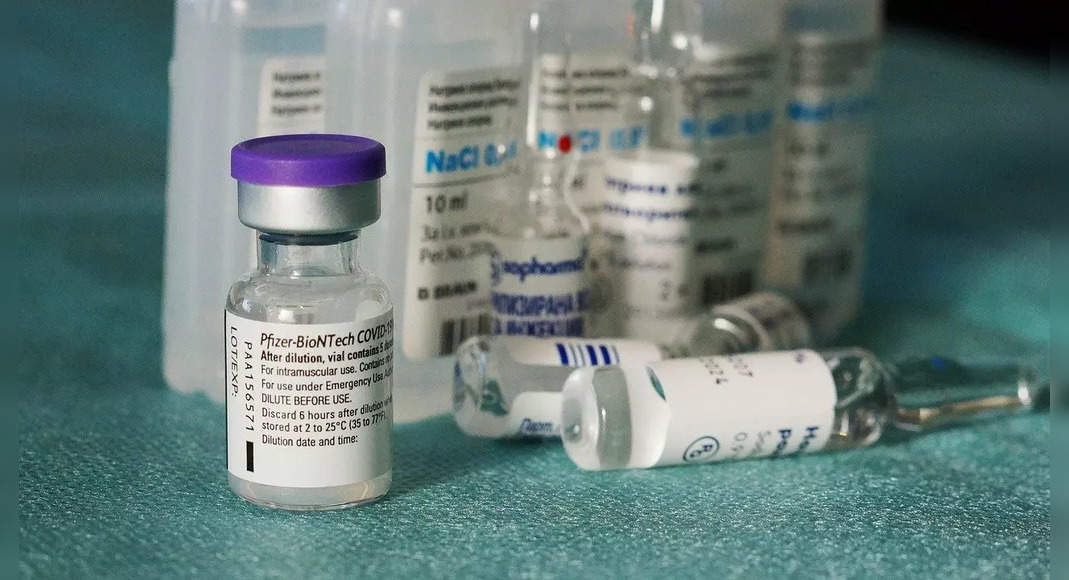
WASHINGTON: The administration of US food and drugs on Monday authorizes the use of the third dose of the Pfizer Vaccine and Bionech Covid-19 for children aged between 12 and 15 years, and narrow the time for all booster shots up to 5 months after the primary dose.
This agency also passed the third shot in children aged 5 to 11 years immunocompromised.
The FDA said it would review the data published and the real world evidence of the security of the booster dose provided by the Israeli Ministry of Health included data from more than 6,300 individuals aged 12 to 15 years who received pfizer shots.
Covid-19 global cases jumped because the Omicron variant and health authorities have warned that their very high transmission can flood many health systems.
Laboratory tests have shown that two doses of Pfizer-Bionontech vaccines and moderna produce low immune responses against omicrons, while booster seems to protect against a very mutative variant.