US health regulators can approve the third Covid-19 shot for adults start at least six months after full vaccination, instead of the eight-month gap that was announced earlier, the Wall Street Journal reported on Wednesday.
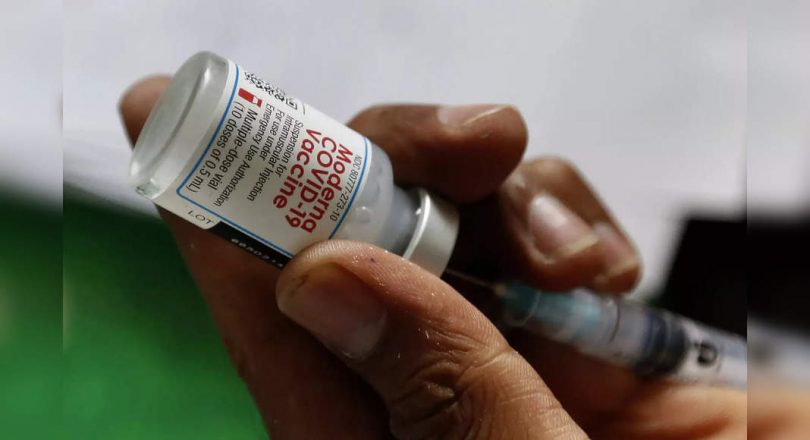
Booster approval for three Covid-19 shots given in the US – produced by Pfizer and Bionontech partners, Moderna Inc.
and Johnson & Johnson – It is estimated that in mid-September, the report said, quoting someone who was familiar with the plan.
Pfizer and Bionech have begun the application process for booster shot approval in people aged 16 years and over, saying it presents an increase in antibodies that are more than three times the corrodavirus.
Earlier this week, US regulators gave full approval to the two-dose vaccine Pfizer.
Moderna said on Wednesday he completed real-time reviews needed for full approval for his Jab to 18 and above.
White House spokesman Jen PSAKi said in his daily briefing that such developments would be under the scope of disease centers and controls and prevention.
CDC said the government’s plan to manage booster’s shots depend on the delayed actions of food and medicines and recommendations for it from the Advisory Committee on immunization practices.
The FDA, however, repeated his statement from last week which said the government prepared to launch a third shot from mid-September to Americans who had a two-dose vaccine road made by modern and pfizer for eight months ago.
The launch will begin if the FDA and CDC decide that the booster is needed, the official said.