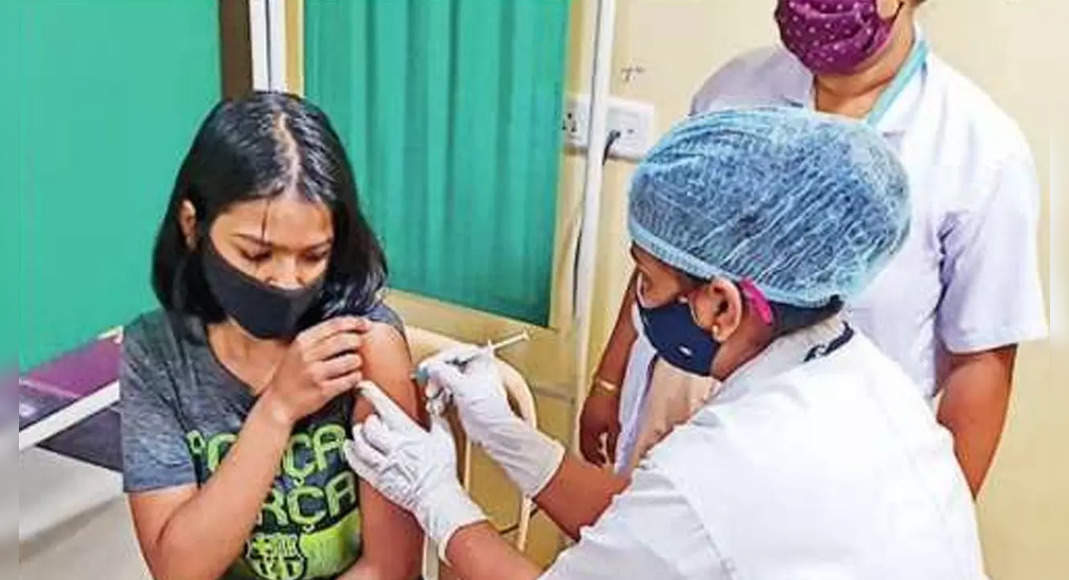
Pune: The Covid Vaccination Program is set to start for the 12-14 age group from February-End, Dr.
N K Arora, Chair of the Covid-19 Working Group of the National Technical Advisory Group concerning Immunization to TOI on Sunday.
Teen vaccinations make impressive steps throughout the country.
As many as 3.31 crore children in the age group 15 to 17 have received their first dose, accounting for coverage of nearly 45%, only 13 days into the drive on January 3 this year.
“We aim to cover all 7.4 crore teens at the bracket aged 15-17 with the first dose in January-end so we can start vaccinating them with the second dose from early February and completing the second dose at the end of February.
We want to start vaccinating children Children between 12 and 14 years since February or early March, “Dr Arora said.
Children aged 12 to 17 are quite like adults, experts say.
“So, the decision was mainly taken to protect adolescents in the group 15-17 first.
After the full vaccination they finished, the government will take policy decisions to include the subsequent age group, which is a 12-14 bracket,” he added.
Experts transfer blows for 12-14 age groups from February NK Arora, Chair of the Covid-19 Working Group of the National Technical Advisory Group on Immunization on Sunday said that the drive will begin for the age group 12-14 from February-END.
Teen immunization is important because they move.
They went to school, college, hanging out and at high risk of gaining infection, especially because of the fast-paced omicron variant of Coronavirus.
This is why the government prioritizes this group from under 18 categories, experts said.
“Extending ambitious vaccination of children is a welcome step.
The government must also consider prioritizing the vaccination of comorbid children in the age group 5-14,” said Dr.
Pramod Jog, former President of the National Pediatric Academy India.
Bharat Biotech’s Covaxin, which is given to teenagers (Group 15-18) has an emergency approval of central use for children in groups 2-18.
Vaccines have been found safe and effective in trial children in the aged bracket 2-18.