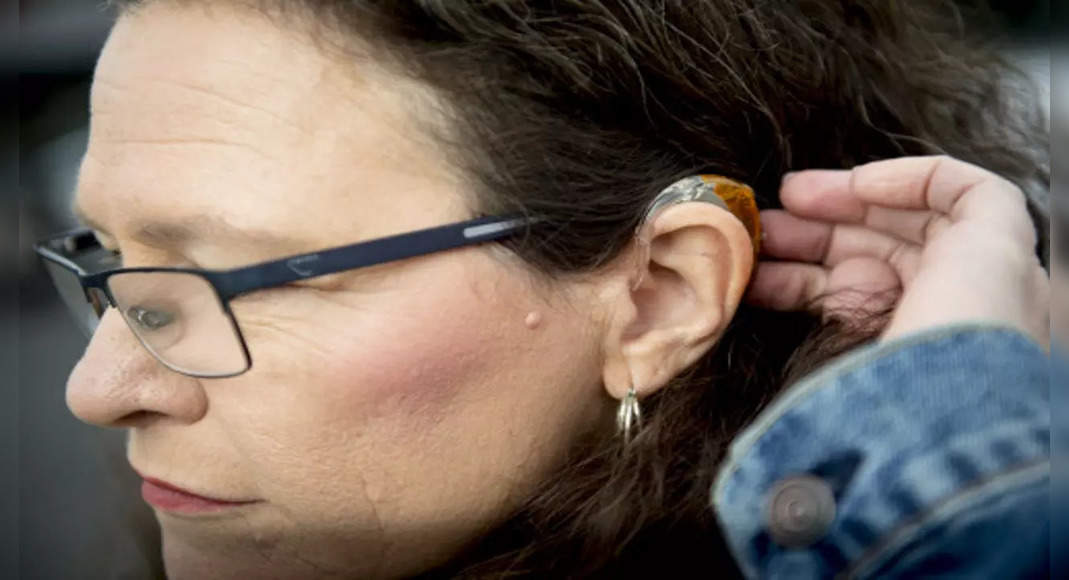
Washington: Health Regulator on Tuesday launched their proposal to allow Americans to buy hearing aids without recipes, a long-awaited steps intended to make the device more accessible by millions of people with hearing problems.
The food and drug administration says the proposed rules will cut red ribbons that currently require hearing exams and recipes for people with mild to moderate hearing loss.
Under the plan, the device can be ordered online or buy over-the-counter at the pharmacy and other retail stores.
This step follows the years of pressure from medical experts and consumer advocates to make devices cheaper and easier to obtain.
More than 37 million Americans, or 15% of adults, have difficulty hearing, according to FDA, but only about one fifth of people who can benefit from hearing aids.
The cost is a big obstacle.
Between the device itself and installation services, Americans can pay more than $ 5,000 to get hearing aids.
Insurance coverage is very limited, and Medicare does not pay hearing aids, only diagnostic tests.
Us.
said on Tuesday that the FDA changed, when resolved, had to spur competition and lower prices.
“Today we open the door for an easier process and a more affordable process,” Xavier Becerra’s human service secretary told reporters.
Agency will take public comments about the proposal for 90 days before completing new rules.
FDA officials will not speculate when new devices will actually hit the store shelf.
Consumer electronics companies for many years have produced low-cost “personal voice amplification” devices, but regulations U.S.
Limiting them from being marketed as a hearing aid and they did not undergo FDA’s review.
The regulator said on Tuesday that new rules would make explicit that the device was not an alternative to FDA-vetted hearing aids.
Companies that market them inappropriately can face federal penalties, such as fine or product seizures.
For their part, hearing aid makers have long argued that professional expertise needed to choose the right device and adjust its settings to work properly.
After the FDA rules apply, traditional manufacturers are expected to start selling cheaper models, direct consumers.
Finally, Advocates predicts the hearing aid market will resemble eye care, where consumers can choose between drug store reading glasses or Bifocals recipes.
Loose regulations will not apply to devices for people with heavy hearing loss or for children.
Also, the agency says the over-the-counter device will be asked to have a volume limit and other steps to help prevent injury.
The company makes excessive hearing aids generally will not be asked to study people.
Instead, they will send applications to the FDA which shows that they meet the standards for the device.
Tuesday’s announcement following the encouragement from the Medical and Congress Committee, which in 2017 instructed the agency to issue a plan for excessive hearing devices in August 2020.
The agency missed the Covid-19 Pandemic time limit.
In addition, the executive order by President Joe Biden earlier this year set a schedule for agents to take action no later than mid-November.