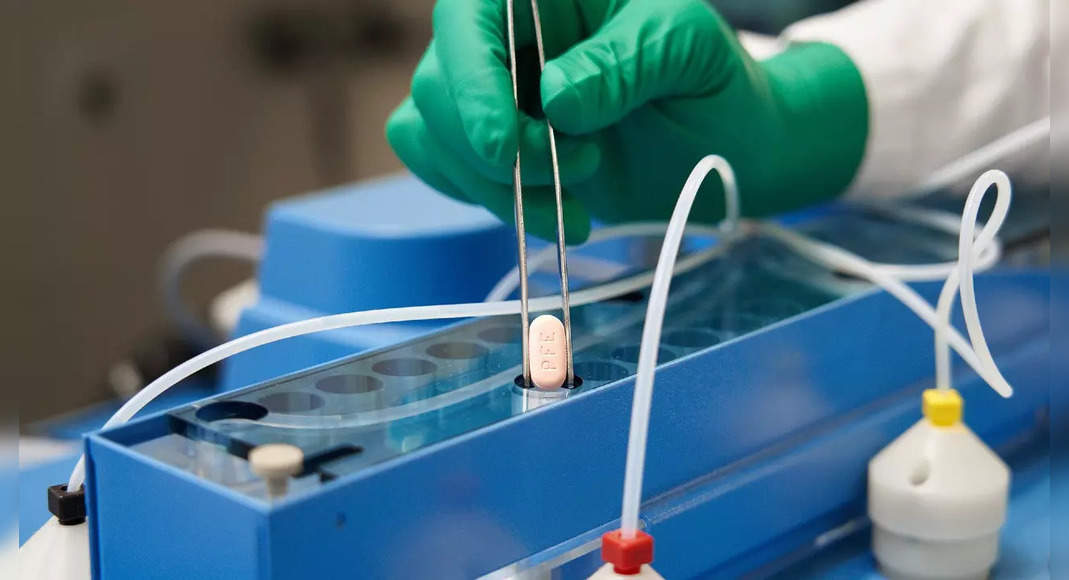
Pfizer Inc.
said on Wednesday, the US food and drug administration ratified the antiviral pill Covid-19, making it first care at home for Coronavirus which is expected to be an important tool in the war against the omicron variant that spreads rapidly.
Data from the clinical trial Pfizer shows the antivirus regimen of two drugs is 90% effective in preventing hospitalization and death in patients with high risk of severe illness.
The new lab data shows the drug maintains its effectiveness of Omicron.
Agencies ratify oral drugs for the treatment of high-risk adult patients and child patients at least 12 years with Covid-19 outside the hospital.
The company said it was ready to start immediate delivery in the US and raised its production projection to 120 million treatment programs from 80 million in 2022.
US government contracts for 10 million Pfizer drug programs valued at $ 530 per course.
Pfizer pills, taken with ritonavir longer antivirus drugs, will be sold under the Paxlovid brand name.
Pills are intended to drink every 12 hours for five days starting shortly after symptoms.
Pfizer said it planned to submit a new drug application with the FDA in 2022 for potential full regulatory approval.