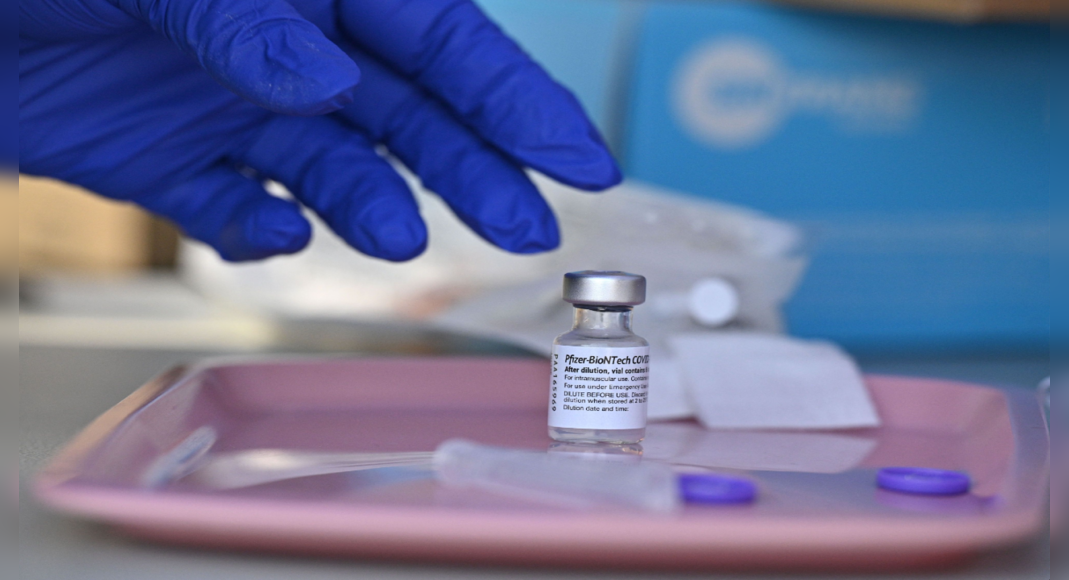
The US Food and Medication Administration (FDA) at the official booster dose Wednesday from the Covid-19 vaccine from Moderna Inc.
and Johnson & Johnson, and said Americans could choose a different shot from the original inoculation as a booster.
The decision to open the way for millions of people in the United States to get additional protection with a highly contagious Delta variant of the virus which causes a breakthrough of infection among some of which are fully vaccinated.
The agency previously the official Booster of the Pfizer Inc Covid-19 vaccine was developed with German partner Bionech SE at least six months after the first half of the shot to increase protection for people aged 65 years and over, those who are at risk of getting severe diseases and they are exposed.
for viruses through their work.
Last week, the advisory panel for the FDA chose to recommend the third round of Moderna vaccine shooting for the same group.
The panel also recommends the second injection of J & J vaccines for all one-dose inoculation recipients at least two months after receiving the first.
Center for FDA and A.S.
For controlling and prevention of disease (CDC) was under pressure to ratify additional shots after the White House announced plans in August for a widespread booster campaign.
Advisory panel meetings include data presentations about mixing vaccines from national institutions A.S.
Health institutions where 458 participants received several combinations of pfizer / bionech shots, modern and J & J.
Data showed that people who initially got the Covid-19 J & J vaccine had a stronger immune response when driven by pfizer or photo shooting, And that “mixing and mixing” booster shots of various types that are safe in adults.
Many countries including the UK have supported a mix-and-match strategy for the widely used astrazeneca PLC vaccine, which is not authorized in the United States but is based on similar viral vector technology as the J & J.
Reuters vaccine reported in June that infectious diseases weighed The need for a modern shooting of Pfizer or vaccine after J & J’s shot.
The CDC Advisory Committee on Thursday will make its recommendations on which group of people should get a modern booster and J & J, which will be used by the agency director to notify the final decision.
About 11.2 million people have so far received a booster dose, according to data from the CDC.